Herbst, C.J., Lopez-Rodriguez, E., Gluhovic, V., Schulz, S., Brandt, R., Timm, S., Abledu, J., Falivene, J., Pennitz, P., Kirsten, K., Nouailles, G., Witzenrath, M., Ochs, M., and Kuebler, W.M. Am J Respir Cell Mol Biol., 2024, 70 (5), 339-350
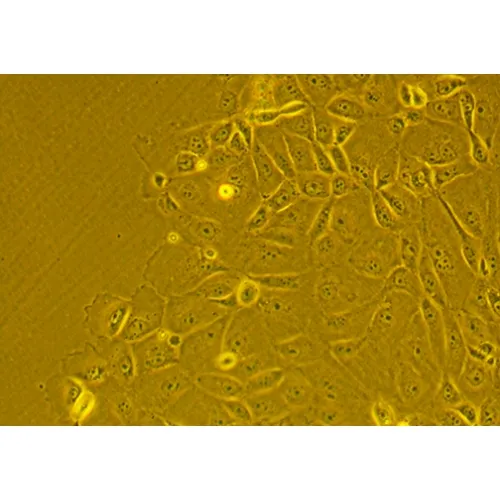
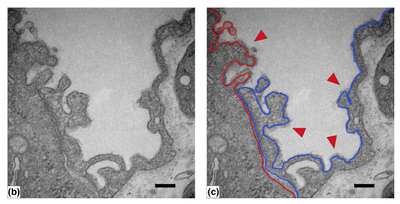
In vitro lung research requires appropriate cell culture models that adequately mimic in vivo structure and function. Previously, researchers have extensively utilized commercially available and easily expandable A549 and NCI-H441 cells which replicate some yet not all features of alveolar epithelial cells. Specifically, these cells are often restricted by terminally altered expression while lacking important alveolar epithelial characteristics. Of late, human primary alveolar epithelial cells (hPAEpC) have become commercially available, but are so far poorly specified. Here, we applied a comprehensive set of technologies to characterize their morphology, surface marker expression, transcriptomic profile, and functional properties. At optimized seeding numbers of 7,500 cells per cm2 and growth at a gas-liquid interface, hPAEpC formed regular monolayers with tight junctions and amiloride-sensitive transepithelial ion transport. Electron microscopy revealed lamellar body and microvilli formation characteristic for alveolar type II cells. Protein and single cell transcriptomic analyses revealed expression of alveolar type I and type II cell markers, yet transcriptomic data failed to detect NKX2-1, an important transcriptional regulator of alveolar cell differentiation. With increasing passage number, hPAEpC transdifferentiated towards alveolar-basal intermediates characterized as SFTPC-, KRT8high and KRT5- cells. In spite of marked changes in transcriptome as a function of passaging, UMAP plots did not reveal major shifts in cell clusters and epithelial permeability was unaffected. The present work delineates optimized culture conditions, cellular characteristics and functional properties of commercially available hPAEpC. hPAEpC may provide a useful model system for studies on drug delivery, barrier function, and transepithelial ion transport in vitro.