Mucci, A., Lopez-Rodriguez, E., Hetzel, M., Liu, S., Suzuki, T., Happle, C., Ackermann, M., Kempf, H., Hillje, R., Kunkiel, J., Janosz, E., Brennig, S., Glage, S., Bankstahl, J.P., Dettmer, S., Rodt, T., Gohring, G., Trapnell, B., Hansen, G., Trapnell, C., Knudsen, L., Lachmann, N., Moritz, T.: Stem Cell Reports. 2018;11(3):696-710.
To access the full article, click here:

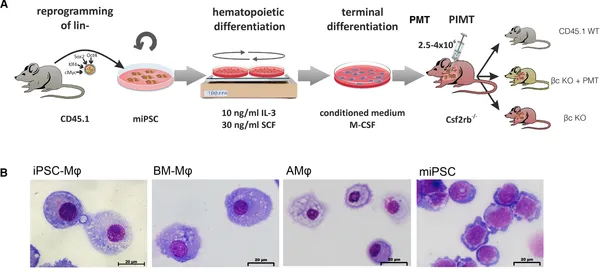
Induced pluripotent stem cell (iPSC)-derived hematopoietic cells represent a highly attractive source for cell and gene therapy. Given the longevity, plasticity, and self-renewal potential of distinct macrophage subpopulations, iPSC-derived macrophages (iPSC-Mφ) appear of particular interest in this context. We here evaluated the airway residence, plasticity, and therapeutic efficacy of iPSC-Mφ in a murine model of hereditary pulmonary alveolar proteinosis (herPAP). We demonstrate that single pulmonary macrophage transplantation (PMT) of 2.5–4 × 106 iPSC-Mφ yields efficient airway residence with conversion of iPSC-Mφ to an alveolar macrophage (AMφ) phenotype characterized by a distinct surface marker and gene expression profile within 2 months. Moreover, PMT significantly improves alveolar protein deposition and other critical herPAP disease parameters. Thus, our data indicate iPSC-Mφ as a source of functional macrophages displaying substantial plasticity and therapeutic potential that upon pulmonary transplantation will integrate into the lung microenvironment, adopt an AMφ phenotype and gene expression pattern, and profoundly ameliorate pulmonary disease phenotypes.